Do you have BACK ACNE that has not responded to standard medications or creams?
You may be a candidate for our clinical research study! If enrolled, you’ll receive up to $600 in compensation for participation!
For more information, please fill out the form below to be contacted by the Cutera Clinic, or reach us directly at (650) 515-1979 or cuteraclinic@cutera.com.
The Cutera Clinic will be in touch to assess your acne condition.
By submitting the image above, you consent to the Cutera Clinic using the image to assess your acne condition for purposes of determining whether you qualify for participation in the treatments.
You can revoke this consent at any time by emailing cuteraclinic@cutera.com, at which time the Cutera Clinic will delete the image you submitted.
This study is IRB approved.
- Ages 16-60
- All genders
- Not pregnant or breastfeeding
- Not participating in another clinical trial
- Willing to stop current acne medications for 2 weeks prior to the start of the study
- Must be willing to receive up to 3 laser treatments
- Must be willing to attend up to 4 on-site follow-up visits
- Other criteria may apply
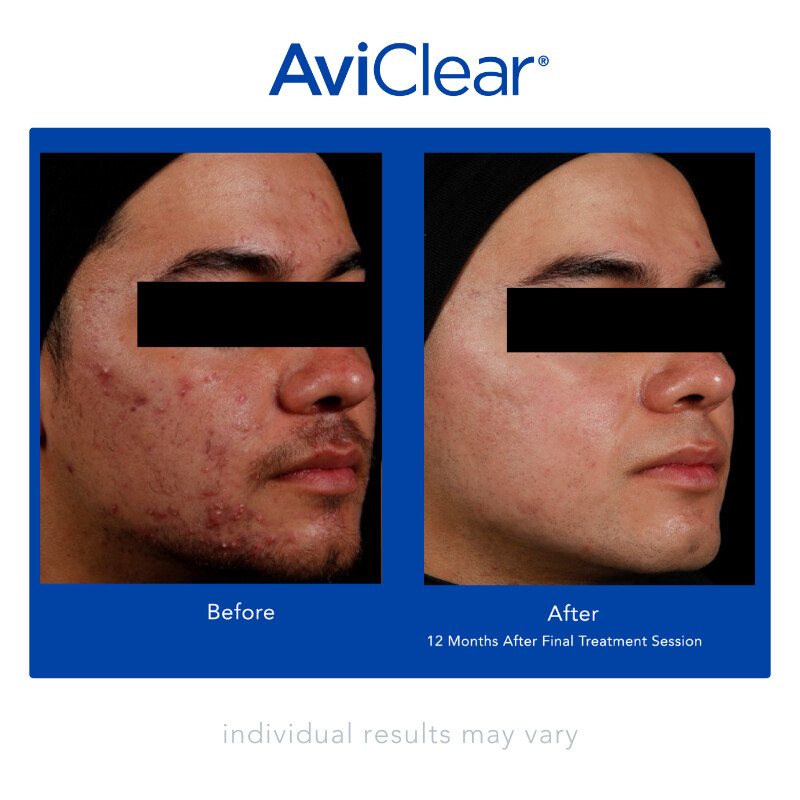
AviClear is a first-of-its-kind laser treatment that specifically targets the source of acne – excess sebum from the sebaceous glands1
AviClear treats active inflammatory acne and helps prevent future acne by suppressing the sebaceous glands.2
AviClear is safe for all skin types and tones, as well as mild, moderate, and severe inflammatory acne.3
The Cutera Clinic is comprised of expert clinical practitioners who help conduct aesthetic and dermatology-based treatments with medical aesthetic devices manufactured and distributed by Cutera.
Cutera is a leading provider of aesthetic and dermatology solutions for practitioners worldwide. For over 25 years, Cutera has strived to improve lives through medical aesthetic technologies that are driven by science and powered through partnerships.
For additional information, contact the Cutera Clinic
Phone: 650-515-1979 | Email: cuteraclinic@cutera.com
3240 Bayshore Blvd. Brisbane, CA 94005
